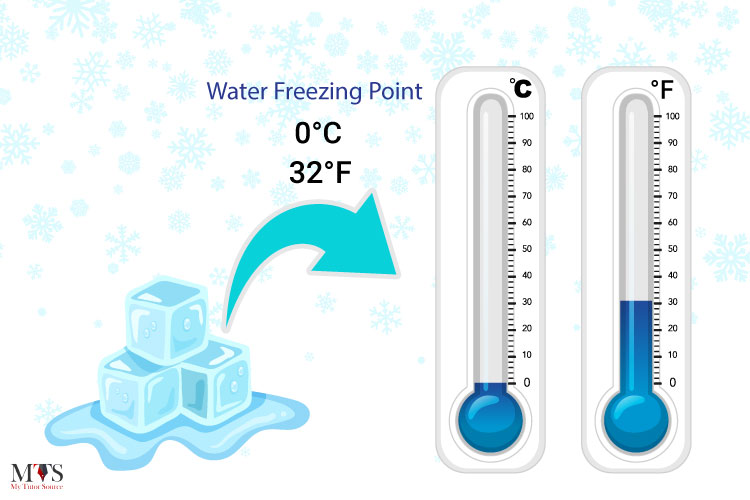
What is freezing point? Water freezes at what temperature? And what is the difference between freezing point and melting point? Let’s find out:
The freezing point of water (liquid) is the temperature at which it transitions to ice (solid). While the melting point of water is when it transitions from solid (ice) to liquid (water). The freezing point of water in Kelvin is 273.2 K while it is 0°C in Celsius. However, the temperature can change if it is supercooled or if there are any substances mixed like impurities in it. This phenomenon is also known as freezing point depression.
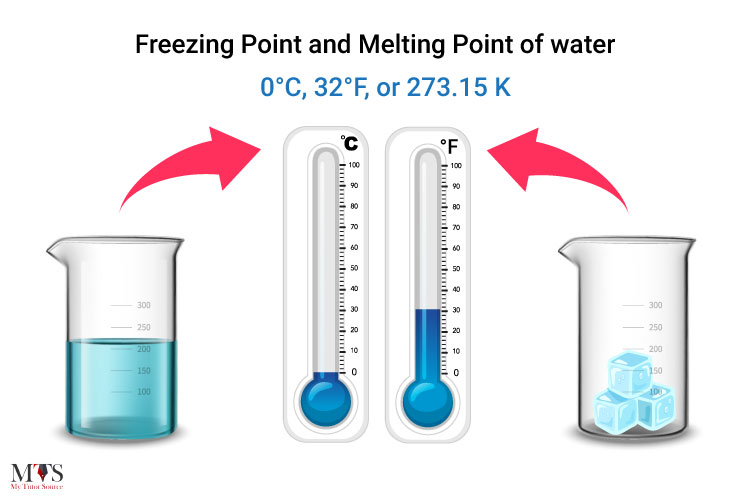
What is the Difference Between Freezing Point and Melting Point?
Both of these terms are often confused together because they’re the same temperatures for practical reasons. So, it is safe to say that the melting point of water is also 0°C, 32°F, or 273.15 K (same as its freezing point).
However, it is important to remember that the freezing point of pure water can also be way too lower than its normal melting point or freezing point. The reason behind this peculiar phenomenon is that it is easy to supercool water because supercooled water does not have any surface defects, air bubbles, or impurities that can allow crystal formation to happen. So, if you pour pure water present in a smooth beaker, it can go to a temperature as low as -40 to -42 °F before it freezes to solid (ice).
What Are The Factors That Influence The Freezing Point Of Water?
Let’s take a look at some of the factors that affect h2o freezing point:
- The types of molecules that make up the structure of a liquid i.e., water can influence its freezing point. In other words when the intermolecular forces that hold these molecules together are stronger then it also makes the freezing point of that liquid high. On the contrary, if the intermolecular forces of attraction that hold the molecules together are weak, then they decrease the freezing point of that liquid. So, in short, it is right to say that the water freezing point is directly proportional to its intermolecular forces.
- Moreover, the freezing point of water can also be influenced by any chemical or physical changes occurring in it. So, for example, if you mix another liquid or soluble substance in it, you may decrease its freezing point.
- In terms of physical changes, you can also alter the freezing point by changing the pressure. Normally, if you reduce the pressure lower than 1atm, you can reduce the temperature at which a liquid freezes. However, in the case of water, the more you increase the pressure, the more its freezing point decreases.
- Other factors that can affect the freezing point of water are particles that do not dissolve in it. For example, substances like pollen or dust can increase the freezing point of water. These particles act as nucleation points and offer the molecules of water a point for attachment to begin the process of crystallization into ice.
And that is all you need to know about the water freezing point in order to understand the concept behind it.
Find Top Tutors in Your Area
Find A Tutor