
What is percentage error? Well, it is the difference between the known or exact value of something and its measured value or approximate value but in percentage form. The percentage error in scientific experiments, to find out the difference between the value obtained from an experiment to its exact true or actual value. So, the difference is measured as the percentage of the exact value, hence, the term percentage error.
As an example in real life, let’s look at a toy machine or a vending machine for soft drinks and make an estimate of how many toys or soft drinks are in the said machines, respectively and then you go ahead and actually count the number of items (toys or soft drinks), then you will be able to find out the difference (AKA the percentage error) in your guess and the actual or true value.
Percent error helps you estimate how far off is your estimate or guess from the actual value of something. Percentage errors can occur because of various reasons like adjustments often made in calculation methods like rounding numbers off, human errors, tool errors, or imprecision of equipment used in an experiment. However, there is a very simple, easy, and straightforward formula to calculate the percentage error. Let’s take a look at it:
Percentage error = [|Approximate value – Exact Value| / Exact value] * 100.

So, the equation for percent error is as follows:
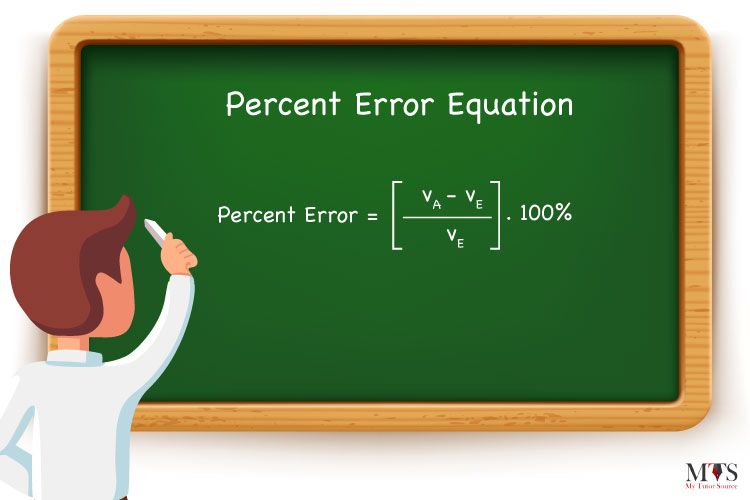
![]()
Where,
= the percent error
VA = the actual value observed
VE = the expected value
Most of the time, percentage error is written as a positive value. However, for chemistry experiments and other sciences, it is important to keep a negative value in case any negative error occurs. And whether an error is negative or positive is also very important. For instance, if you are doing an experiment and you did not expect to get a positive percentage error when comparing actual yield to theoretical yield in a chemical reaction, you will realize the need to have a positive value as well because it could have provided you with clues about potential problems with the experiment or unaccounted reactions.
Yes, it can. Percentage error can be a negative value as well, especially in chemistry, there are frequent negative percentage errors. In chemistry, for example, a chemical reaction between two or more than two substances may have an already previously established final yield. So, in such reactions and experiments, it is vital for the scientists performing it to report the accuracy of their results in order to find out the direction of their error. So, they keep a negative error as well to indicate a lower yield.
Follow the steps listed below to find out percentage error in any observation or experiment:
Step 1: Find out the value of your error by subtracting one of your values from your other value. The order to subtraction does not matter if you are keeping the sign in your equation. However, if you use the minus sign in your equation then you need to find out your error by subtracting the exact value from the measured value of your experiment.
Step 2: Follow the next step by dividing the difference of the two values (AKA the error value) by the exact or the known value (do not confuse it with your experimental or measured value. After the division, you will be left with a decimal number.
Step 3: Lastly, all you have to do is multiply the decimal value you got in your previous step with 100 to get your answer in a percentage value.
Step 4: Finally, you need to add the percent sign (%) with your calculated value to find out your percentage error.
If your answer is near zero, then your approximation is quite close to the true value of the experiment you are performing. The percentage error formula is super important if you want to find out how precise your calculations are. So, keep this formula, equation, and detailed steps in mind and you are good to go.