
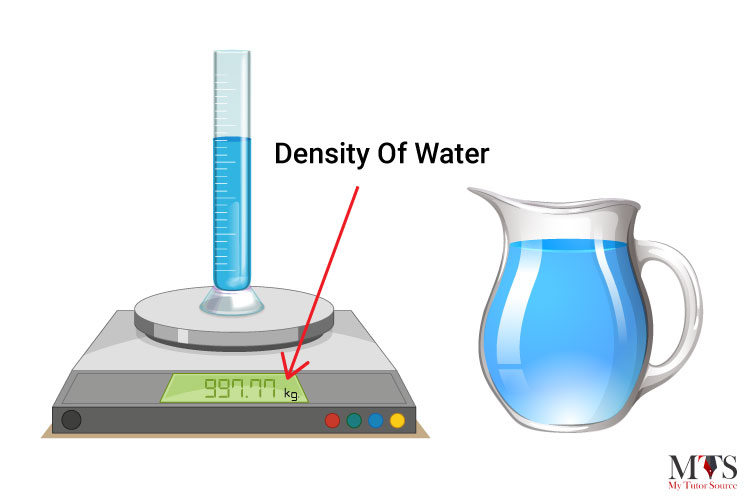
The density of water is defined as the weight of water (in grams) per unit volume (in milliliter) and it relies on its temperature. The value that we usually use in mathematical calculations is 1 gram per milliliter (1 g/ml) which is also known as the fluid density of water. The common unit of density of water is 1 gram per cubic centimeter (1 g/cm³).
The density of 100% pure water is actually less than 1 g/cm³. Plus, you can supercool water; it will still remain a liquid even though it is way below its freezing point.
As mentioned before, the density of water can change with temperature. So, here’s a handy table for you that tells you the density of water pounds per cubic foot or density of water in pounds along with others:
| Temperature (°F/°C) | Weight (pounds/ft3) | Density (grams/cm3) |
| 32°F/0°C | 62.416 | 0.99987 |
| 39.2°F/4.0°C | 62.424 | 1.00000 |
| 40°F/4.4°C | 62.423 | 0.99999 |
| 50°F/10°C | 62.408 | 0.99975 |
| 60°F/15.6°C | 62.366 | 0.99907 |
| 70°F/21°C | 62.300 | 0.99802 |
| 80°F/26.7°C | 62.217 | 0.99669 |
| 90°F/32.2°C | 62.118 | 0.99510 |
| 100°F/37.8°C | 61.998 | 0.99318 |
| 120°F/48.9°C | 61.719 | 0.98870 |
| 140°F/60°C | 61.386 | 0.98338 |
| 160°F/71.1°C | 61.006 | 0.97729 |
| 180°F/82.2°C | 60.586 | 0.97056 |
| 200°F/93.3°C | 60.135 | 0.96333 |
| 212°F/100°C | 59.843 | 0.95865 |
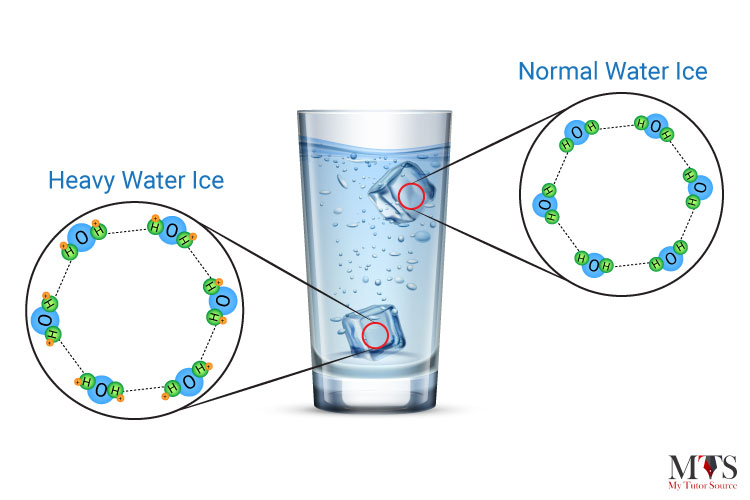
Ice of heavy water sinks in water owing to its higher density because deuterium atoms replace hydrogen items in heavy water. Deuterium is an isotope of hydrogen in which there is one electron, one proton, and one neutron. When a neutron is added to an atom of hydrogen, it makes deuterium 10.6% denser than your normal water. Therefore, heavy ice sinks in normal water and floats in heavy water.
The simplest way to find the density of a liquid is with a hydrometer. A hydrometer has a weighted bulb and a cylindrical stem with lines on it that represent how far the weighted bulb sinks in the liquid. The lower it sinks, the lower is the density, the higher it floats, the higher the density. The lines are calibrated by using the hydrometer in a standard liquid with known density (usually water with a specific gravity of 1.000 at 4°C).
Or you can use the volume and mass:
The reason why water is at its maximum density at 4°C is that at 4°C both opposing effects are in balance.
The density of normal water is 22° C at room temperature and it is 997.77 in kg/m3.
As we already know, water is at its maximum density at 4°C. When compared to water, ice has a lower density which is the reason why it floats. When it freezes its density decreases by about 9%.
The reason why water does not possess an absolute density is because water’s density changes with temperature.
And there you have everything you need to know about the density of water, the factors affecting it, and how to calculate it. Best of luck!