

Acid and bases can be found everywhere in our daily life products. Let it be the lemon and vinegar (acids) we add to our eatables or the soap and bleach (bases) we use to wash our clothes with; there are countless examples of acids and bases around us.
Acids are basically the substances with a pH level below 7, and they are capable of donating their hydrogen ions (H+), whereas the bases, on the other hand, are the substances with pH levels ranging from 7-14, and these compounds are capable of donating their hydroxyl ion (OH–).
Are you working on your exam? You are at the right place! Read below all about the detailed aspects of acids, bases, and their reactions.
Acid is a substance that is usually a liquid and contains a hydrogen ion that is ready to be donated, and it produces certain salts upon reaction with other substances. The acids are found in our common food items, including citrus fruits.

Acids have been defined by three different theories, with all explaining distinct features of acids. Here we have discussed the general ideas behind the three theories of acids.
According to this theory, acid is defined as a substance that yields electronically charged ions, i.e., Hydrogen, in an aqueous solution.
According to this theory of acids, acid is a substance that donates a proton in an aqueous solution.
As per the Lewis theory of acid, an acid is a substance that accepts electrons when present in an aqueous solution.
Here are some of the common examples of acids and how they are used based on their chemical and physical properties.
Let’s discuss in depth the daily life uses of these acid examples.
The carbonic acid with the chemical formula (H2CO3) is a type of acid that is mainly composed of Hydrogen, carbon, and oxygen. Normally at temperatures above −80 °C, this acid tends to decompose.
The sodas, bubbly drinks, and carbonated drinks that we normally drink all contain carbonic acid as a chief constituent. Other than this, it is also present in groundwater, rainwater, and certain plants.
Other common uses of carbonic acid include:
The acetic acid with the chemical formula (CH3COOH) is also known more commonly as ethanoic acid. Besides being a main component of the vinegar acid and being responsible for its classic odor, acetic acid is also used in other areas such as printing processes and textiles production.
These are some other relevant uses of the acetic acid:
The citric acid with the chemical formula HOC(CH₂CO₂H)₂ is considered as a colorless organic acid, and it can easily be found in some fruits and vegetables of our daily uses, including lemon, oranges, and other citrus fruits. It is known to have a sour-tasting nature.
These are some other common uses of citric acid:
Hydrochloric acid with the chemical formula (H⁺ Cl⁻) is, also sometimes known as muriatic acid, does not exhibit any particular color, but it is considered a strong and corrosive acid. In our digestive system, hydrochloric acid is known to be a major component of gastric juice and further facilitates the breakdown and digestion of foods.
These are some of the other common uses of hydrochloric acid.
Sulphuric acid with the chemical formula (H2SO4) is sometimes known as hydrogen sulfate. Being oil-based, colorless, and corrosive, it is majorly used on lead-acid-based batteries.
These are some of the other common uses of sulphuric acid:
Nitric acid with the chemical formula (HNO3) is a colorless and extremely corrosive acid. Its fuming property makes it the main constituent in the production of explosives.
Here are some of the other common uses of nitric acid:
These are some of the common physical properties associated with acids.

These are some of the common chemical properties that are associated with acids.
This neutralization reaction can be seen when hydrochloric acid- HCl (acid) reacts with sodium hydroxide- NaOH (base), and the resulting product is Sodium chloride- NaCl (table salt) and water- H2O
This can be seen in the example when magnesium bicarbonate-MgCO3 (Carbonate) reacts with two molecules of hydrochloric acid- 2HCl (acid) the resulting product is magnesium chloride- MgCl2 (salt), Carbon Dioxide (CO2), and water (H2O)
Based on the level of acidity, there can be a few categories of acids. Let’s discuss how these categories differ based on the level of acidity.
Strong- When the acid has the ability to break off entirely, and as a result, it gives off a maximum number of ions or protons, then the acid is considered a strong acid. Some of the examples of a strong acid include Hydrochloric acid, Nitric acid, and Sulfuric acid.
Weak- On the other hand, when an acid does not dissociate entirely in a solution and hence it is not able to give off all of its ions or protons, then the acid is categorized as a weak acid. Some examples of weak acids include acetic acid, oxalic acid, and benzoic acid.
From our homes to industries, the use of acids is induced in our daily lives one way or the other. Here we have listed some of the major ways acids are generally used in our everyday life.

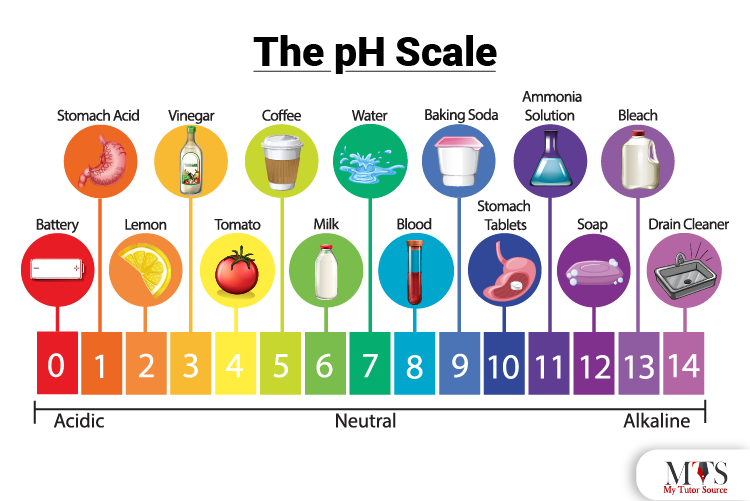
The potential of Hydrogen or pH is known as the hydrogen concentration in a solution. The level of hydrogen concentration in a solution is used to indicate the pH level of the solution hence determining the acidity or basicity of it. The pH table ranges from 0 to 14. In this range, 7 is considered as a neutral solution such as water.
From 7 to 14, the range is used to measure the basicity of a solution, with 14 being a strong base, whereas 0 to 7 indicates the acidity of a solution, with 0 or 1 being a strong acid.
Here are some of the examples of strong and weak acids and bases with their respective pH levels.
Acids
Some of the acid names with their pH level are
Bases
Some of the base names with their pH levels are
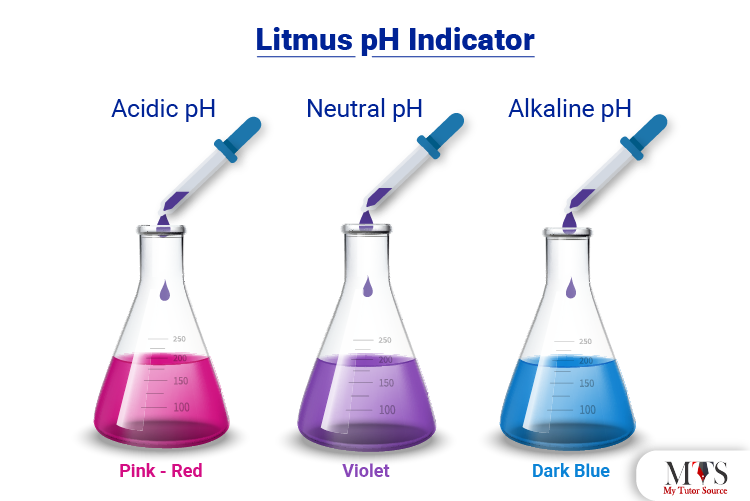
Litmus paper is a paper that has been prepared specially from treatment with a mixture of several dyes.
The purpose of the litmus paper is to indicate whether a particular solution is an acid or a base. In order to perform the indicator test, a small amount of the sample solution is added to the paper. The paper then changes its color to indicate the nature of the solution being acidic or basic.
If the litmus paper turns red, then the solution is found to be acidic.
If the litmus paper turns blue, then the solution is found to be basic.
If the litmus paper turns purple, then the solution is found to be neutral.
A neutral substance is one that does not show any property related to an acid or a base. These solutions have a balanced concentration of hydrogen and hydroxyl ions in them. Their pH usually stands at 7. Being neutral, they do not exhibit any particular kind of change as an indicator.
A common example of a neutral substance with no acidic or basic quality is water and human saliva with a pH of 7.
When a neutral substance gains a pH value above 7, it becomes basic, whereas if its pH level comes down in a reaction, it becomes acidic.

A base is defined as any substance that produces hydroxyl ions (OH–) in an aqueous solution and holds the ability to neutralize an acid with a pH greater than 7.

According to the different aspects of bases, there are three distinct theories that are associated with bases. Here are some of the theories that have been used to describe a base:
According to this theory, a base is defined as a substance that is capable of donating a hydroxyl ion (OH–) when present in an aqueous solution.
According to the Bronsted Lowry Theory of bases, a base can be defined as a substance that is capable of accepting a proton when present in an aqueous solution.
According to this theory of base, a base is defined as a substance that can donate an electron pair when it is present in an aqueous solution.
Based on the difference of their physical and chemical properties, there are several examples of bases as mentioned below:
Here are some of the daily life uses of these bases mentioned above
Sodium hydroxide with a chemical formula NaOH, also known as caustic soda, is an odorless base. It is used in the production of soaps, and because of its corrosive nature, it is also used in the production of explosive products.
These are some of the other common uses of sodium hydroxide:
Potassium hydroxide with the chemical formula (KOH) is an odorless and white inorganic substance, and it is considered a highly strong base with multiple applications in the industrial sector.
These are some of the common uses of potassium hydroxide:
Calcium hydroxide with the chemical formula (Ca(OH)2), also known as slaked lime, forms a product known as lime water when dissolved in an aqueous solution. It is usually a colorless white powder widely being used in industrial processes.
Here are some of the common uses of calcium hydroxide:
Lithium hydroxide with the chemical formula (LIOH) is a white solid inorganic compound that results from a chemical reaction between lime and lithium carbonate. Lithium hydroxide is usually classified as a strong base.
Here are some of the common uses of lithium hydroxide:
Zinc hydroxide with the chemical formula Zn(OH)₂ is considered an amphoteric natured inorganic compound that is usually reactive to bases and acids. With notable uses in the medical industry, here are some common uses associated with zinc hydroxide:
Rubidium hydroxide with the chemical formula (RbOH) is usually a plain colorless solid compound, but commercially, it is available in aqueous form. As Rubidium Hydroxide is a strong base, it is considered highly corrosive.
Here are some of the common uses of rubidium hydroxide
Here are some of the physical properties of bases:

Here are some of the chemical properties that are associated with bases:
On the level of basicity in a substance, bases are divided into a few categories. Some of them have been discussed here:
Strong- When a base is present in an aqueous solution, and it is able to completely dissociate in it, A strong base is also known to be a very good proton acceptor. Some examples of strong bases include Sodium hydroxide, lithium hydroxide, and potassium hydroxide.
Weak- A weak base is not a very good proton acceptor. Other than this, when a base is present in an aqueous solution and it does not dissociate completely, it is regarded as a weak base because the solution does not have a lot of concentration of hydroxyl ions from the base. Some of the examples of a weak base include zinc hydroxide, ammonia, and lead hydroxide.
From soaps to electric batteries, we use bases in our daily life, and here we have discussed some of the general uses of bases in our daily lives:

DIFFERENCE BETWEEN ACID AND BASE | |
| ACID | BASE |
BASED ON THEORIES ARRHENIUS: Acid is a chemical substance that gives off hydrogen (H) ions in an aqueous solution. BRONSTED-LOWRY: Acid is a substance that donates protons when present in an aqueous solution. LEWIS: Acid is a substance that accepts electrons when present in an aqueous solution. | BASED ON THEORIES ARRHENIUS: Base is a chemical substance that gives off hydroxyl ions (OH) in an aqueous solution. BRONSTED-LOWRY: Base is a substance that accepts a proton when present in an aqueous solution. LEWIS: Base is a substance that is capable of donating an electron pair in an aqueous solution. |
pH The pH of an acid lies below 7 on the pH scale. | pH The pH of a base lies between 7 and 14 on a pH scale. |
Color of litmus paper Acid turns the litmus paper into red. | Color of litmus paper Base turns litmus paper into blue. |
Taste Acids are known to have a sour taste. | Taste Bases are known to have a bitter taste. |
Ions in aqueous solution When present in an aqueous solution, Acids give off Hydrogen (H+) ions. | Ions in aqueous solution When present in an aqueous solution, bases give off Hydroxyl ions (OH-) |
Acidity strength The strength of an acidic solution is determined by the presence of Hydrogen ion concentration in the solution. | Basicity strength The strength of a basic solution is determined by the presence of hydroxyl ions concentration in an aqueous solution. |
State of existence Acids are usually present in the form of solid, liquid, and gaseous states. | State of existence Except for a few bases like ammonia that are present in the gas state, all bases usually exist in a solid-state. |
pH of a strong acid The acid with a low pH is considered a strong acid. An acid having a pH of 1 is the strongest acid. | pH of a strong base The base with high pH is considered a strong base. A base having a pH of 14 is the strongest base. |
Phenolphthalein When present in an acidic solution, the phenolphthalein is found to be turned colorless. | Phenolphthalein When present in a basic solution, the phenolphthalein is found to be turned into pink color. |
Methyl orange indicator When present in an acidic solution, methyl orange shows the color red. | Methyl orange indicator When present in a basic solution, methyl orange shows the color yellow. |
Common uses Some common uses of acids are
| Common uses Some common uses of bases include
|
Examples of acids Some common examples of acids include
| Examples of bases Some common examples of bases include
|
Acids and bases are chemical substances that give off hydrogen and hydroxyl ions respectively in an aqueous solution. Acids have a pH value below the level of 7, whereas bases have a pH value above the level of 7 and range till 14.
Do you wish to learn and explore all the detailed areas of chemistry? Our private chemistry tutors can help you navigate it all thoroughly and clearly.